A chemist mixed two substances
together: a blue powder with no smell
and a colorless liquid...

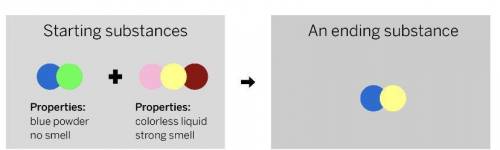
A chemist mixed two substances
together: a blue powder with no smell
and a colorless liquid with a strong
smell. Their repeating groups of atoms
are shown above on the left. After they
were mixed, the chemist analyzed the
results and found two substances. One
ending substance had the repeating
group of atoms shown above on the
right.
Is the ending substance the same
substance as the blue powder? What
happened to the atoms of the starting
substances when the ending
substances formed? Be sure to explain
your answers to both of these

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1

Chemistry, 23.06.2019 04:00
What two categories of toxins were present in the air at dish,texas as a result of the gas pipelines that pass through the area
Answers: 1

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2

You know the right answer?
Questions




Mathematics, 09.07.2019 07:00



Mathematics, 09.07.2019 07:00

SAT, 09.07.2019 07:00

Computers and Technology, 09.07.2019 07:00

Mathematics, 09.07.2019 07:00

Mathematics, 09.07.2019 07:00

Mathematics, 09.07.2019 07:00

Mathematics, 09.07.2019 07:00



Spanish, 09.07.2019 07:00

Mathematics, 09.07.2019 07:00

Spanish, 09.07.2019 07:00

Mathematics, 09.07.2019 07:00



