
Chemistry, 07.05.2021 04:10 krystalhurst97
Calculations:
Show your calculations for each of the following. Remember, calculations should follow rules for significant figures.
Write the balanced chemical equation for the reaction you are performing.
Mg(s) + O2(g) → MgO(s)
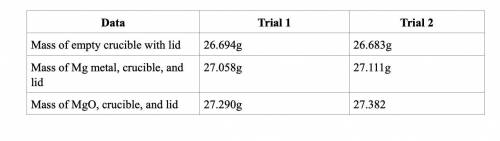
Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of Mg, crucible, and lid (row 2 in the chart) to find the mass of magnesium for each trial.
Trial 1: 0.364
Trial 2: 0.428
Subtract the mass of the crucible and lid (row 1 in the chart) from the total mass of MgO, crucible, and lid (row 3 in the chart) to find the mass of magnesium oxide for each trial. This is the actual yield of magnesium oxide for each trial.
Trial 1: 0.596
Trial 2: 0.699
Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for each trial.
Trial 1:
Trial 2:
Determine the percent yield of MgO for your experiment for each trial.
Trial 1:
Trial 2:
Determine the average percent yield of MgO for the two trials.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 07:00
In the cathode ray tube experiment, j. j. thomson passed an electric current through different gases inside a cathode ray tube in the presence of an electric field. in which two ways did this experiment change scientists’ understanding of the atom?
Answers: 2

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1
You know the right answer?
Calculations:
Show your calculations for each of the following. Remember, calculations should foll...
Questions


Mathematics, 18.07.2019 00:30

Mathematics, 18.07.2019 00:30






Business, 18.07.2019 00:30


Mathematics, 18.07.2019 00:30

Mathematics, 18.07.2019 00:30







Mathematics, 18.07.2019 00:30



