
Chemistry, 20.05.2021 01:30 idjfjcjs584
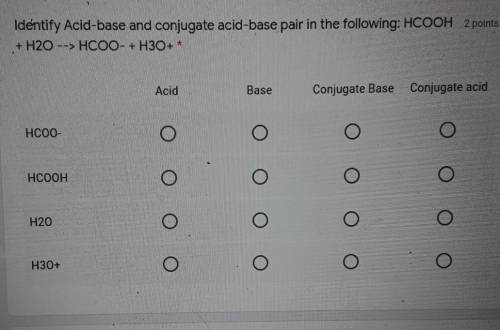
Identify Acid-base and conjugate acid-base pair in the following: HCOOH + H20 --> HCOO + H3O+ *

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 23:30
With the largest atoms and the smallest number of valence electrons and with the smallest atoms and the greatest number of valence electrons are the most reactive. a. nonmetals; metals b. nonmetals; transition elements c. transition elements; metals d. metals; nonmetals
Answers: 3
You know the right answer?
Identify Acid-base and conjugate acid-base pair in the following: HCOOH + H20 --> HCOO + H3O+ *...
Questions



Mathematics, 30.01.2020 04:05


Mathematics, 30.01.2020 04:05


History, 30.01.2020 04:05

English, 30.01.2020 04:05


Mathematics, 30.01.2020 04:40



Mathematics, 30.01.2020 04:40

Chemistry, 30.01.2020 04:40

Mathematics, 30.01.2020 04:40




English, 30.01.2020 04:40

History, 30.01.2020 04:40



