
Chemistry, 24.05.2021 21:50 jasonkindred21
NEED HELP ASSP!!
(If you could show all the steps or work to get the answers that would be greatly appreciated)
62. A compound was found to contain 49.98 g of carbon and 10.47 g of hydrogen. The molar
mass of the compound is 58.12 g/mol. Determine the molecular formula.
63. A colorless liquid
composed of 46.68% nitrogen and 53.32% oxygen has a molar mass of
60.01 g/mol. What is the molecular formula?
64. When an oxide of potassium is decomposed, 19.55 g of K and 4.00 g of O are obtained.
What is the empirical formula for the compound?
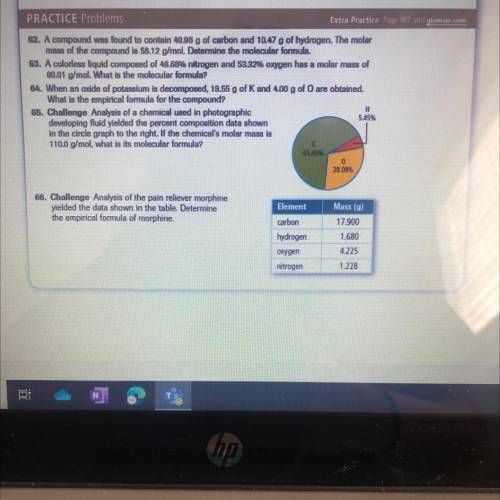
65. Challenge Analysis of a chemical used in photographic
developing fluid yielded the percent composition data shown
in the circle graph to the right. If the chemical's molar mass is
110.0 g/mol, what is its molecular formula?
66. Challenge Analysis of the pain reliever morphine
yielded the data shown in the table. Determine
the empirical formula of morphine.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:40
You may expect bonds between two atoms which each have n covalent lonic metallic hydrogen
Answers: 2

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2


Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1
You know the right answer?
NEED HELP ASSP!!
(If you could show all the steps or work to get the answers that would be greatly...
Questions



















Computers and Technology, 15.10.2019 17:10



