Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2


Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

You know the right answer?
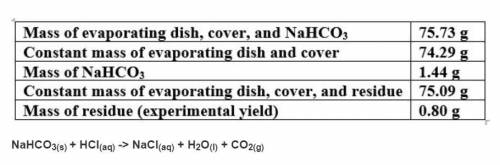
Based on a theoretical yield of 1.00 grams NaCl, calculate the mass in grams of hydrochloric acid yo...
Questions

Biology, 08.04.2021 14:00

Social Studies, 08.04.2021 14:00

Social Studies, 08.04.2021 14:00



English, 08.04.2021 14:00

Biology, 08.04.2021 14:00

World Languages, 08.04.2021 14:00

Mathematics, 08.04.2021 14:00

Computers and Technology, 08.04.2021 14:00


Physics, 08.04.2021 14:00

Mathematics, 08.04.2021 14:00

Mathematics, 08.04.2021 14:00

Social Studies, 08.04.2021 14:00


Engineering, 08.04.2021 14:00

Mathematics, 08.04.2021 14:00