
Chemistry, 29.05.2021 22:00 brianna8739
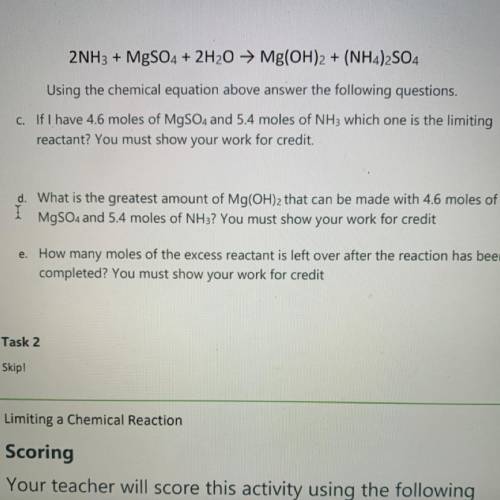
2NH3 + MgSO4 + 2H2O → Mg(OH)2 + (NH4)2SO4
Using the chemical equation above answer the following questions.
C. If I have 4.6 moles of MgSO4 and 5.4 moles of NH3 which one is the limiting
reactant? You must show your work for credit.
d. What is the greatest amount of Mg(OH)2 that can be made with 4.6 moles of
MgSO4 and 5.4 moles of NH3? You must show your work for credit
e. How many moles of the excess reactant is left over after the reaction has been
completed? You must show your work for credit

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
2NH3 + MgSO4 + 2H2O → Mg(OH)2 + (NH4)2SO4
Using the chemical equation above answer the following qu...
Questions

Mathematics, 29.01.2020 22:48

History, 29.01.2020 22:48

Mathematics, 29.01.2020 22:48


Chemistry, 29.01.2020 22:48

Business, 29.01.2020 22:48


English, 29.01.2020 22:48

Business, 29.01.2020 22:48


Chemistry, 29.01.2020 22:48

Mathematics, 29.01.2020 22:48

Mathematics, 29.01.2020 22:48

Business, 29.01.2020 22:48


Mathematics, 29.01.2020 22:48


Advanced Placement (AP), 29.01.2020 22:48

Mathematics, 29.01.2020 22:48

Biology, 29.01.2020 22:48



