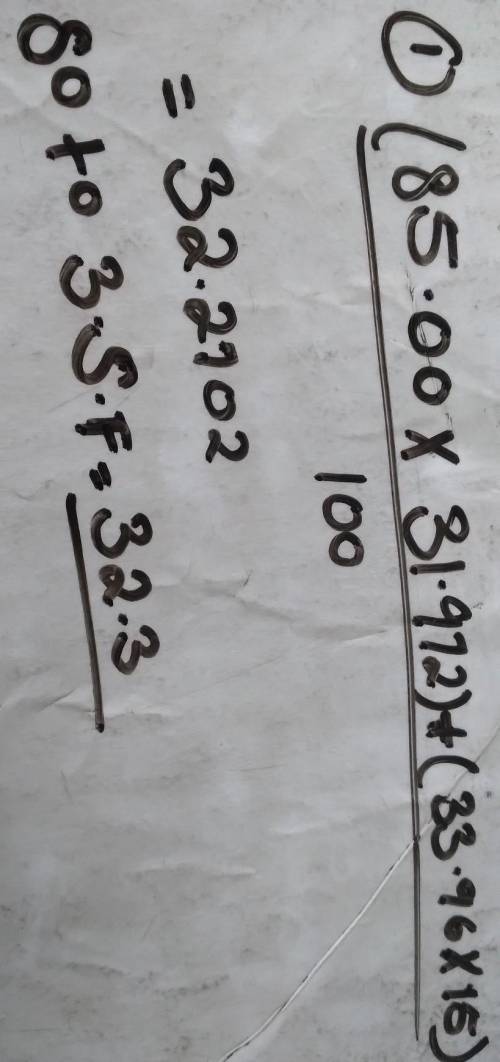Chemistry, 03.06.2021 21:20 birdman37361
Calculate the atomic mass of Nitrogen if the two common isotopes of Nitrogen have masses of 31.972 amu (85.00 % abundance) and 33.96 amu (15.00% abundance)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 23.06.2019 04:31
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1

Chemistry, 23.06.2019 08:30
Kelly has come up with an explanation for why her sister is sometimes in a good mood and other times in a bad mood. she speculates that it is based on the hours of sleep her sister got the previous night. this explanation for her sister's behaviors is an example of a(n)
Answers: 3
You know the right answer?
Calculate the atomic mass of Nitrogen if the two common isotopes of Nitrogen have masses of 31.972 a...
Questions

Chemistry, 29.01.2020 05:40



English, 29.01.2020 05:40


Mathematics, 29.01.2020 05:40






English, 29.01.2020 05:40


Mathematics, 29.01.2020 05:40

Mathematics, 29.01.2020 05:40




Mathematics, 29.01.2020 05:40