
Chemistry, 09.06.2021 03:00 blkrosesss
Calculate the number of milliliters of 0.587 M NaOH required to precipitate all of the Fe3 ions in 197 mL of 0.654 M FeCl3 solution as Fe(OH)3. The equation for the reaction is: FeCl3(aq) 3NaOH(aq) Fe(OH)3(s) 3NaCl(aq)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 11:00
When hydrochloric acid reacts with potassium hydroxide solution, the following reaction occurs. hcl (aq) + koh (aq) h2o (l) + kcl (aq) the reaction gives off heat energy, so it is an reaction.
Answers: 1

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1
You know the right answer?
Calculate the number of milliliters of 0.587 M NaOH required to precipitate all of the Fe3 ions in 1...
Questions


Chemistry, 26.10.2020 22:00

Mathematics, 26.10.2020 22:00


Mathematics, 26.10.2020 22:00

French, 26.10.2020 22:00


Mathematics, 26.10.2020 22:00

Mathematics, 26.10.2020 22:00

Mathematics, 26.10.2020 22:00

Social Studies, 26.10.2020 22:00

Mathematics, 26.10.2020 22:00

Mathematics, 26.10.2020 22:00


Mathematics, 26.10.2020 22:00

Biology, 26.10.2020 22:00


Advanced Placement (AP), 26.10.2020 22:00

Mathematics, 26.10.2020 22:00

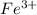 ions in 197 mL of 0.654 M
ions in 197 mL of 0.654 M 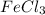 solution as
solution as 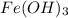 .
.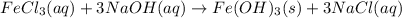
 Volume (in L)
Volume (in L)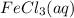 reacts with 3 moles of NaOH(aq). Hence, moles of
reacts with 3 moles of NaOH(aq). Hence, moles of 


