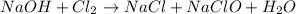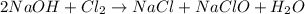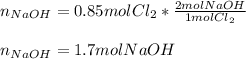Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 20:00
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1
You know the right answer?
If we have 1.23 mol of NaOH in solution and 0.85 mol of Cl2 gas is available to react, which one is...
Questions

Physics, 05.05.2021 07:20


Biology, 05.05.2021 07:20



Mathematics, 05.05.2021 07:20

Mathematics, 05.05.2021 07:20

Physics, 05.05.2021 07:20



Mathematics, 05.05.2021 07:20


Spanish, 05.05.2021 07:20






Mathematics, 05.05.2021 07:20

Biology, 05.05.2021 07:20