
Chemistry, 15.06.2021 17:50 mbarber3994
A helium tank has a volume of 13.0 L at a pressure of 26.5 atm. What is the volume of the same amount of helium at a pressure of 1.25 atm?

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

You know the right answer?
A helium tank has a volume of 13.0 L at a pressure of 26.5 atm. What is
the volume of the same amou...
Questions












Computers and Technology, 13.12.2019 17:31






Computers and Technology, 13.12.2019 17:31


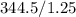 , and the final pressure (P2) is 1.25. Using Boyle's Law, we plug in the values into the equation.
, and the final pressure (P2) is 1.25. Using Boyle's Law, we plug in the values into the equation. 

