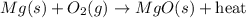Chemistry, 18.06.2021 07:40 adamkinney6110
When heated, magnesium combines readily with excess oxygen in the air to produce magnesium oxide, as shown in the following unbalanced equation.
Mg (s) + O2 (g) → MgO (s) + heat
What two types of reactions could this chemical equation be classified as?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:00
An atom of which element reacts with an atom of hydrogen to form a bond with the greatest degree of polarity ?
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2
You know the right answer?
When heated, magnesium combines readily with excess oxygen in the air to produce magnesium oxide, as...
Questions

Mathematics, 27.05.2021 05:10



Mathematics, 27.05.2021 05:10

Chemistry, 27.05.2021 05:10




Mathematics, 27.05.2021 05:10

Mathematics, 27.05.2021 05:10

Mathematics, 27.05.2021 05:10


History, 27.05.2021 05:10


English, 27.05.2021 05:10

Physics, 27.05.2021 05:10


Mathematics, 27.05.2021 05:10

Physics, 27.05.2021 05:10