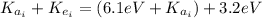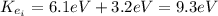The first excited state of a particular atom in a gas is 6.1 eV above the ground state. A moving electron collides with one of these atoms, and excites the atom to its first excited state. Immediately after the collision the kinetic energy of the electron is 3.2 eV. What was the kinetic energy of the electron just before the collision

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 14:30
Select the word from the list that best fits the definition the nuclear family into which a person is born or adopted.
Answers: 2


Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
The first excited state of a particular atom in a gas is 6.1 eV above the ground state. A moving ele...
Questions






Mathematics, 03.12.2020 06:30

History, 03.12.2020 06:30

Mathematics, 03.12.2020 06:30

Mathematics, 03.12.2020 06:30

Mathematics, 03.12.2020 06:30


Social Studies, 03.12.2020 06:30


Business, 03.12.2020 06:30

Mathematics, 03.12.2020 06:30

History, 03.12.2020 06:30

Biology, 03.12.2020 06:30

Mathematics, 03.12.2020 06:30


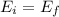
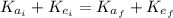 (1)
(1)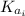 : is the initial kinetic energy of the atom
: is the initial kinetic energy of the atom 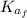 : is the final kinetic energy of the atom = 6.1 eV +
: is the final kinetic energy of the atom = 6.1 eV + 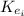 : is the initial kinetic energy of the electron =?
: is the initial kinetic energy of the electron =? 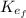 : is the final kinetic energy of the electron = 3.2 eV
: is the final kinetic energy of the electron = 3.2 eV