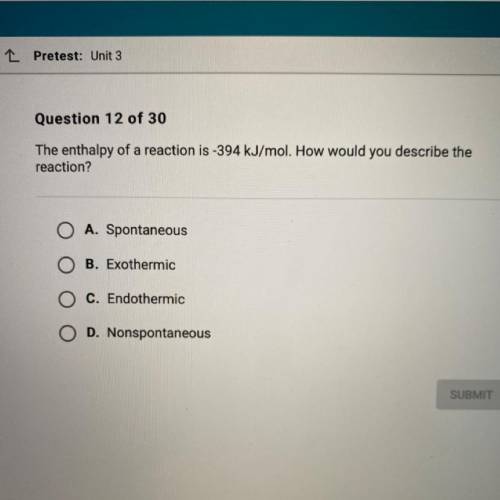
The enthalpy of a reaction is -394 kJ/mol. How would you describe the
reaction?
A. Spontaneou...


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 21:00
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1

Chemistry, 23.06.2019 04:00
How much energy is required to vaporize 2 kg of copper? a 4730 kj b 207kj c 9460 kj d 414kj
Answers: 1

Chemistry, 23.06.2019 04:31
Pls i will do pls imma diewhat forms white light? (4 points)a. combination of all wavelengths of ultraviolet light b. combination of all wavelengths of visible lightc. absorption of electromagnetic waves d. absorption of infrared rays
Answers: 2
You know the right answer?
Questions

Mathematics, 07.07.2019 02:30

History, 07.07.2019 02:30


Mathematics, 07.07.2019 02:30

Biology, 07.07.2019 02:30



Mathematics, 07.07.2019 02:30

Mathematics, 07.07.2019 02:30

Mathematics, 07.07.2019 02:30

Mathematics, 07.07.2019 02:30

Mathematics, 07.07.2019 02:30

Biology, 07.07.2019 02:30

Mathematics, 07.07.2019 02:30




Mathematics, 07.07.2019 02:30