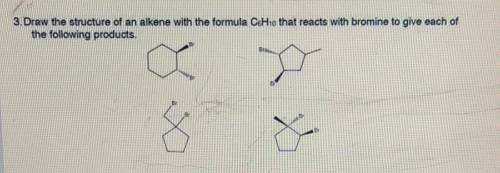
Chemistry, 02.07.2021 23:40 genyjoannerubiera
Draw the structure of the alkene with the molecular formula C6H10 that reacts with Br2 to give this compound.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

You know the right answer?
Draw the structure of the alkene with the molecular formula C6H10 that reacts with Br2 to give this...
Questions




Geography, 12.09.2019 23:20

Mathematics, 12.09.2019 23:20

History, 12.09.2019 23:20


Mathematics, 12.09.2019 23:20


Mathematics, 12.09.2019 23:20







Mathematics, 12.09.2019 23:20