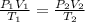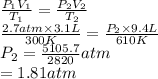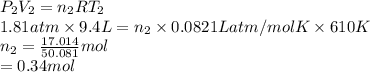A cylinder contains 3.1 L of oxygen at 300 K and 2.7 atm. The gas is heated, causing a piston in the cylinder to move outward. The heating causes the temperature to rise to 610 K and the volume of the cylinder to increase to 9.4 L.
How many moles of gas are in the cylinder?
Express your answer using two significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and gas called? identify which way they are transitioning
Answers: 2


Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
A cylinder contains 3.1 L of oxygen at 300 K and 2.7 atm. The gas is heated, causing a piston in the...
Questions



Biology, 02.03.2020 06:37

Mathematics, 02.03.2020 06:37



Mathematics, 02.03.2020 06:37


English, 02.03.2020 06:38



Mathematics, 02.03.2020 06:38


Mathematics, 02.03.2020 06:38


Computers and Technology, 02.03.2020 06:38

Social Studies, 02.03.2020 06:38


Mathematics, 02.03.2020 06:38

History, 02.03.2020 06:38

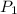 = 2.7 atm,
= 2.7 atm, 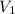 = 3.1 L,
= 3.1 L, 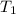 = 300 K
= 300 K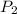 = ?,
= ?, 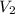 = 9.4 L,
= 9.4 L, 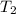 = 610 K
= 610 K