
Chemistry, 21.07.2021 01:00 QueenZenobia
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different concentration of reactant was used during each trial, whereas the other factors were kept constant.
Which of the following statements explains which trial has a lower concentration of the reactant?
A: Trial 1, because the average rate of the reaction is lower.
B: Trial 1, because this reaction lasted for a longer duration than Trial 2.
C: Trial 2, because this reaction was initially fast and later slowed down.
D: Trial 2, because the volume of product formed per unit time was higher.
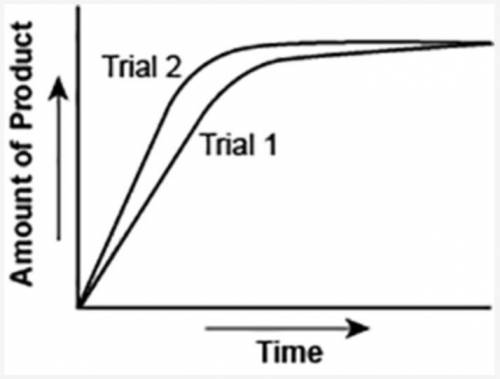

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1


Chemistry, 22.06.2019 23:00
What is the energy in joules of a mole of photons associated with visible light of wavelength 486 nm?
Answers: 3

Chemistry, 23.06.2019 11:40
Which of the following would have the lowest average kinetic energy
Answers: 1
You know the right answer?
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different...
Questions

Mathematics, 27.01.2021 08:50

Mathematics, 27.01.2021 08:50

Mathematics, 27.01.2021 08:50

Mathematics, 27.01.2021 08:50

Chemistry, 27.01.2021 08:50

Mathematics, 27.01.2021 08:50


Mathematics, 27.01.2021 08:50

Mathematics, 27.01.2021 08:50



English, 27.01.2021 09:00



Mathematics, 27.01.2021 09:00



Advanced Placement (AP), 27.01.2021 09:00

Mathematics, 27.01.2021 09:00

English, 27.01.2021 09:00



