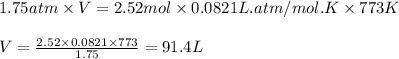Chemistry, 21.07.2021 20:00 imstressed
What volume of water is produced when 38.5 g of ethanol reacts with oxygen at 500°C at 1.75 atm? CH3CH2OH(g) + 3 O2(g)→ 2 CO2(g) + 3 H2O(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

You know the right answer?
What volume of water is produced when 38.5 g of ethanol reacts with oxygen at 500°C at 1.75 atm?
CH...
Questions



Mathematics, 02.03.2021 05:10




Mathematics, 02.03.2021 05:10

Mathematics, 02.03.2021 05:10


English, 02.03.2021 05:10

Mathematics, 02.03.2021 05:10

Mathematics, 02.03.2021 05:10



Mathematics, 02.03.2021 05:10

Mathematics, 02.03.2021 05:10




Mathematics, 02.03.2021 05:20

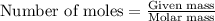 ......(1)
......(1)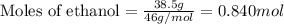
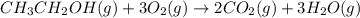
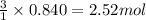 of water
of water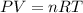 .......(2)
.......(2)![500^oC=[500+273]K=773K](/tpl/images/1397/4323/8da9a.png)