
Chemistry, 06.08.2021 04:40 rogersdeloris1ovgm3b
Acids and Bases Titration
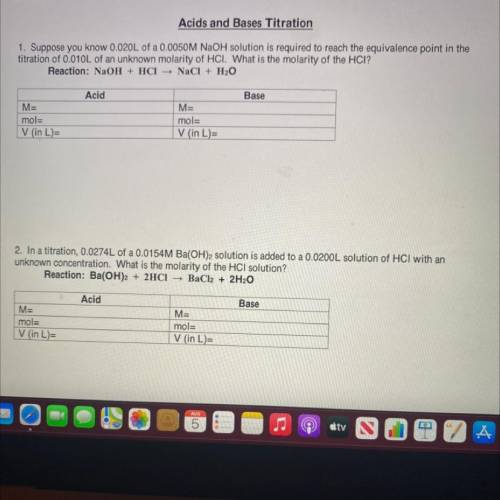
1. Suppose you know 0.020L of a 0.0050M NaOH solution is required to reach the equivalence point in the
titration of 0.010L of an unknown molarity of HCI. What is the molarity of the HCI?
Reaction: NaOH + HCl NaCl + H2O
Acid
Base
M=
mol=
V (in L)=
M=
mol=
V (in L)=

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1
You know the right answer?
Acids and Bases Titration
1. Suppose you know 0.020L of a 0.0050M NaOH solution is required to reac...
Questions

Mathematics, 08.11.2020 15:10

Computers and Technology, 08.11.2020 15:10

Chemistry, 08.11.2020 15:10

Mathematics, 08.11.2020 15:10

Mathematics, 08.11.2020 15:10

Mathematics, 08.11.2020 15:10

English, 08.11.2020 15:10




English, 08.11.2020 15:20

English, 08.11.2020 15:20

Mathematics, 08.11.2020 15:20

Social Studies, 08.11.2020 15:20

Biology, 08.11.2020 15:20


Mathematics, 08.11.2020 15:20

Advanced Placement (AP), 08.11.2020 15:20





