
Chemistry, 20.08.2021 08:20 adaneri1234
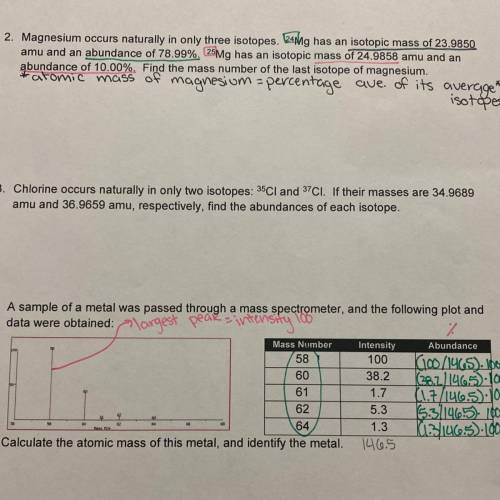
Magnesium occurs naturally in only three isotopes. ^24 Mg has an isotopic mass of 23.9850 amu and an abundance of 78.99%. ^25 Mg has an isotopic mass of 24.9858 amu and an abundance of 10.00%. Find the mass number of the last isotope of magnesium. Can someone please explain how to solve this? *done mine the notes*

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

You know the right answer?
Magnesium occurs naturally in only three isotopes. ^24 Mg has an isotopic mass of 23.9850 amu and an...
Questions








Geography, 06.11.2019 22:31


Spanish, 06.11.2019 22:31


Biology, 06.11.2019 22:31

Chemistry, 06.11.2019 22:31




Arts, 06.11.2019 22:31

History, 06.11.2019 22:31




