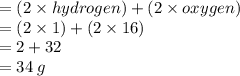Chemistry, 27.08.2021 16:20 cutegirl0987
Hich formula can be used to calculate the molar mass of hydrogen peroxide (H2O2)? (5 points)
Select one:
a. molar mass of H + molar mass of O
b.2 x molar mass of H + molar mass of O
c. molar mass of H + 2 x molar mass of O Incorrect
d.2 x molar mass of H + 2 x molar mass of O
Which of the following statements best defines the actual yield of a reaction? (5 points)
Select one:
a. The amount of product measured after a reaction
b. The ratio of measured yield over theoretical yield
c. The maximum amount of product that can be obtained Incorrect
d. The ratio of measured yield over stoichiometric yield

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 09:30
Which formula can be used to calculate the molar mass of hydrogen peroxide
Answers: 1

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2
You know the right answer?
Hich formula can be used to calculate the molar mass of hydrogen peroxide (H2O2)? (5 points)
Selec...
Questions

Spanish, 20.09.2019 23:30




Physics, 20.09.2019 23:30





History, 20.09.2019 23:30

Mathematics, 20.09.2019 23:30

History, 20.09.2019 23:30


Health, 20.09.2019 23:30



Social Studies, 20.09.2019 23:30