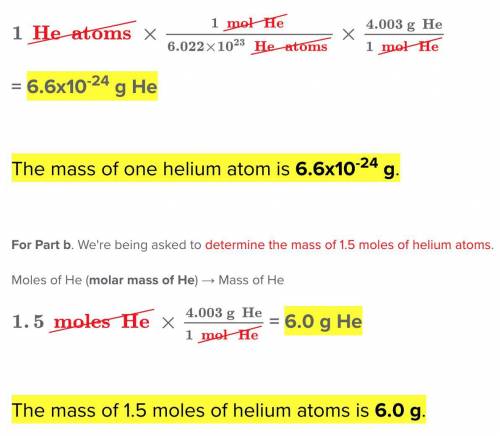
Chemistry, 05.09.2021 15:30 TropicalFan
What is the mass of helium atom whose atomic weight is 4.003 g/mol?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1


Chemistry, 22.06.2019 18:00
What amount of heat is exchanged when 106.2 grams of substance y goes from a liquid at 35 degrees celsius to a solid at the same temperature? melting point of substance y = 35 degrees c; δhvaporization = 3.67 j/mol; δhfusion = 3.30 j/mol. mwsubstance y = 28.22 g/mol. −12.4 j −3.51 x 102 j 1.24 x 101 j 351 j
Answers: 1

Chemistry, 22.06.2019 18:20
Categorize them by metal, nonmetal, in periodic tableductilenon-ductilemalleableoften gain electrons easilygood conductorpoor conductorcan be liquidselements
Answers: 2
You know the right answer?
What is the mass of helium atom whose atomic weight is 4.003 g/mol?...
Questions

Advanced Placement (AP), 21.07.2019 20:30


Mathematics, 21.07.2019 20:30


Mathematics, 21.07.2019 20:30

Mathematics, 21.07.2019 20:30



Mathematics, 21.07.2019 20:30


Mathematics, 21.07.2019 20:30

Biology, 21.07.2019 20:30

Mathematics, 21.07.2019 20:30

Chemistry, 21.07.2019 20:30




Biology, 21.07.2019 20:30

History, 21.07.2019 20:30

Mathematics, 21.07.2019 20:30