

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2

Chemistry, 21.06.2019 23:00
Which statement describes covalent bases? they have hydroxide ions. they produce hydrogen ions. they are often amines. they are named the same as ionic compounds.
Answers: 3

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
A nuclear fission reaction has mass difference between the products and the reactants of 0.0284 amu....
Questions

Physics, 08.11.2019 20:31

History, 08.11.2019 20:31



Mathematics, 08.11.2019 20:31

Health, 08.11.2019 20:31

Social Studies, 08.11.2019 20:31

History, 08.11.2019 20:31

Chemistry, 08.11.2019 20:31


Mathematics, 08.11.2019 20:31

Biology, 08.11.2019 20:31

History, 08.11.2019 20:31


History, 08.11.2019 20:31

History, 08.11.2019 20:31


Mathematics, 08.11.2019 20:31

English, 08.11.2019 20:31

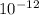
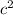
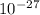 kg = 4.714x
kg = 4.714x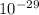 kg
kg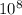
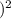 = 4.24 x
= 4.24 x 

