
Chemistry, 26.09.2021 15:50 breezyalanah
The subatomic particle that makes each of these isotopes the same is the _. each of these isotopes has _ protons.


Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 23.06.2019 00:30
Ok, so i have 2 questions. try to answer them both: (the topic is fire) 1) how can you represent the chemical reaction of fire? 2) what kind of bond is formed in this chemical reaction
Answers: 3

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1
You know the right answer?
The subatomic particle that makes each of these isotopes the same is the _. each of these isotopes h...
Questions

Chemistry, 30.09.2019 22:30




Mathematics, 30.09.2019 22:30

Computers and Technology, 30.09.2019 22:30


Mathematics, 30.09.2019 22:30

Health, 30.09.2019 22:30


History, 30.09.2019 22:30

English, 30.09.2019 22:30




Biology, 30.09.2019 22:30



Biology, 30.09.2019 22:30

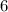 protons in each of these atoms.
protons in each of these atoms. 

