Electrochemistry is important in many aspects of daily life.
i. Define voltaic cell.
ii. Fi...

Chemistry, 26.09.2021 17:10 isabellemdeakin
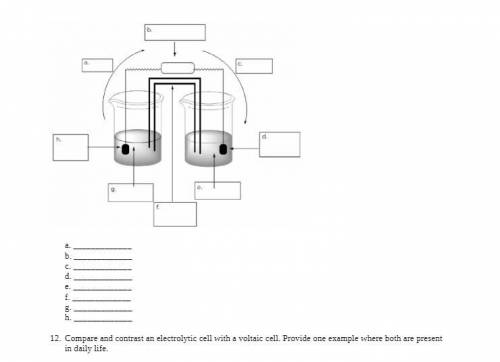
Electrochemistry is important in many aspects of daily life.
i. Define voltaic cell.
ii. Fill in the blanks for the drawing of a voltaic cell that’s made with copper/copper (II) nitrate (E° =
0.34 V) and zinc/zinc (II) nitrate (E° = –0.76 V). Briefly explain the role of the salt bridge.
iii. Using the equation E°cell = E°cathode – E°anode, calculate the overall cell potential for the cell in step b.
Be sure to show all steps completed to arrive at the answer

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 23.06.2019 10:40
Aliquid solution can be made select all that apply. dissolving solids into liquids, mixing liquids, dissolving gas solutes into liquids , mixing gases, mixing solids
Answers: 3

Chemistry, 23.06.2019 20:30
If 4.88 grams of zn react with 5.03 grams of s8 to produce 6.02 grams of zns, what are the theoretical yield and percent yield of this reaction? be sure to show the work that you did to solve this problem.unbalanced equation: zn + s8 yields zns
Answers: 3
You know the right answer?
Questions








Mathematics, 04.12.2019 00:31

Computers and Technology, 04.12.2019 00:31













