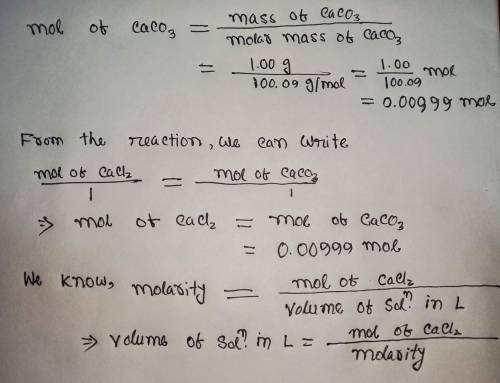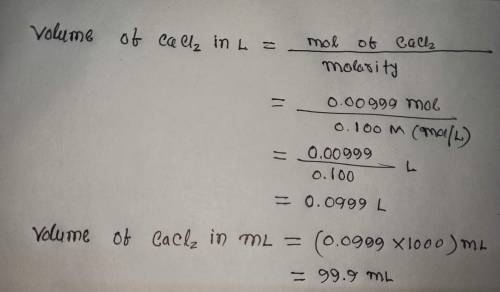Calculate the volume (in mL) of 0.100 M Na, CO3 needed to produce 1.00 g of
CaCO3(s)
. There...

Chemistry, 28.09.2021 03:10 hammackkatelyn60
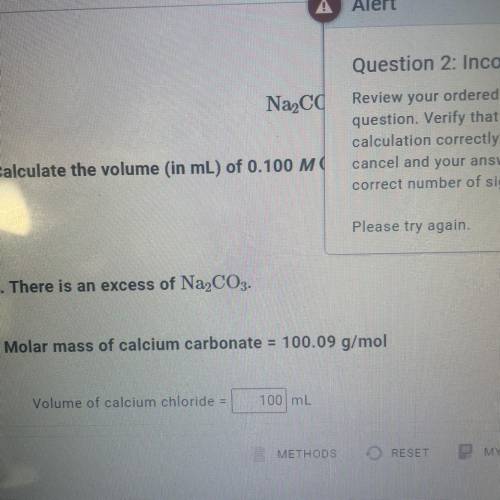
Calculate the volume (in mL) of 0.100 M Na, CO3 needed to produce 1.00 g of
CaCO3(s)
. There is an excess of CaCl2.
Molar mass of calcium carbonate = 100.09 g/mol
*The answer is not 100ml or 10ml. Somehow the rounding up is not working well.*

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

You know the right answer?
Questions


Mathematics, 06.12.2021 19:20

History, 06.12.2021 19:20

Mathematics, 06.12.2021 19:20


Business, 06.12.2021 19:20



History, 06.12.2021 19:20

Chemistry, 06.12.2021 19:20

Computers and Technology, 06.12.2021 19:20


History, 06.12.2021 19:20


Mathematics, 06.12.2021 19:20

Mathematics, 06.12.2021 19:20

Mathematics, 06.12.2021 19:20


Spanish, 06.12.2021 19:20

Mathematics, 06.12.2021 19:20