Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 12:30
Avariable that is not being directly tested during an experiment should be
Answers: 1

You know the right answer?
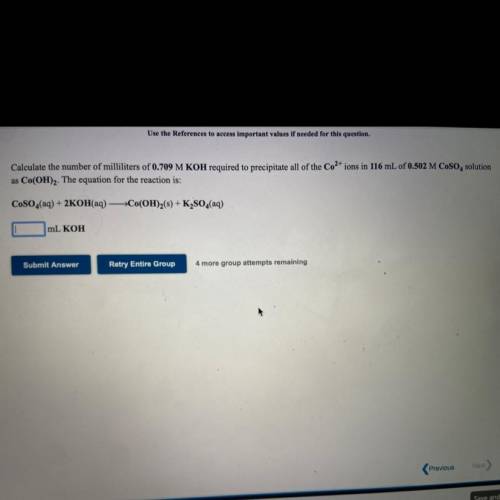
Calculate the number of milliliters of 0.709 M KOH required to precipitate all of the Co2+ ions in 1...
Questions



Mathematics, 30.08.2020 01:01


Physics, 30.08.2020 01:01

Mathematics, 30.08.2020 01:01



Mathematics, 30.08.2020 01:01



Physics, 30.08.2020 01:01




English, 30.08.2020 01:01



Mathematics, 30.08.2020 01:01