
ATOMS-
1. an atom is the smallest part of a pure substance which retains the _ of that pure substance
COMPOUNDS-
2. atoms of each element are essentially the _
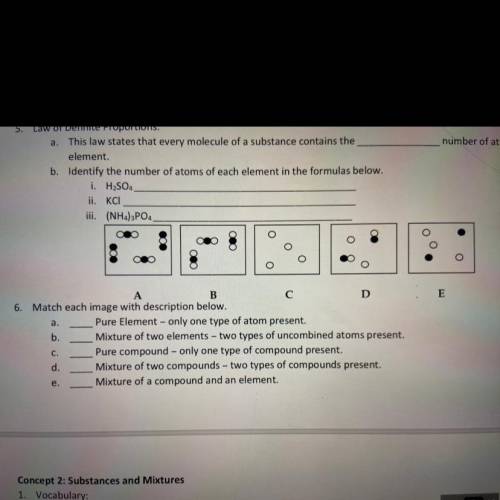
LAW OF DEFINITE PROPORTIONS-
3. this law states that every molecule of a substance contains the _ number of atoms of each element
SOLUTIONS-
4. Solutions are special types of mixtures which are _ so they often can be confused as pure substances
STATES OF MATTER-
5. how would you describe the change in arrangement of particles as heat energy and temperature increases?
6. why does the temperature remain constant during a phase change even though the substance is absorbing heat energy?
7. (number six only)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Find the protons, electrons and neutrons for strontium with a mass of 83
Answers: 1

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 09:50
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
ATOMS-
1. an atom is the smallest part of a pure substance which retains the _ of that pure substa...
Questions





Social Studies, 15.10.2019 18:30











Social Studies, 15.10.2019 18:30

History, 15.10.2019 18:30

History, 15.10.2019 18:30


Mathematics, 15.10.2019 18:30



