
Chemistry, 11.10.2021 18:40 donnafranks2003
An excess of sodium carbonate, Na, CO3, in solution is added to a solution containing 17.87 g CaCl2. After performing the experiment, 13.19 g of calcium carbonate, CaCO3, is produced. Calculate the percent yield of this reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1



Chemistry, 23.06.2019 13:30
These traits describe either a chemical or a nuclear reaction. which statements describe a nuclear reaction? check all that apply. involves the loss, gain, or sharing of electrons may involve a change in total mass involve relatively low energy changes occur outside the nucleus involve very high-energy changes involve changes in nuclides when decay occurs
Answers: 1
You know the right answer?
An excess of sodium carbonate, Na, CO3, in solution is added to a solution containing 17.87 g CaCl2....
Questions



Mathematics, 18.02.2020 19:22





Computers and Technology, 18.02.2020 19:22

World Languages, 18.02.2020 19:22



Mathematics, 18.02.2020 19:22


History, 18.02.2020 19:23



Mathematics, 18.02.2020 19:23

History, 18.02.2020 19:23

Mathematics, 18.02.2020 19:23

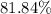 .
.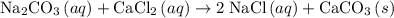 .
.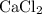 , as well as those in the product of interest,
, as well as those in the product of interest, 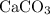 :
: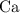 :
: 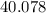 .
.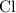 :
: 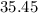 .
. :
: 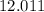 .
. :
:  .
.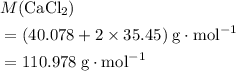 .
.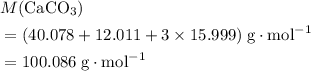 .
.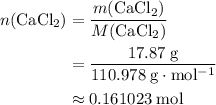 .
.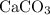 ) are both
) are both  . Thus:
. Thus: .
. 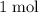 of
of 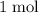 of
of 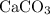 in this experiment:
in this experiment: .
. .
. , calculate the percentage yield of this experiment:
, calculate the percentage yield of this experiment: .
.

