
Chemistry, 23.10.2021 19:10 spazzinchicago
Aluminum (AI) and silver tarnish (Ag²S) yield pure silver (Ag) in an aluminum sulfide solution. What are the reactants and products in this reaction?
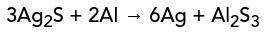

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2


Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2
You know the right answer?
Aluminum (AI) and silver tarnish (Ag²S) yield pure silver (Ag) in an aluminum sulfide solution.
Wh...
Questions

Mathematics, 19.07.2019 08:30









History, 19.07.2019 08:30


Social Studies, 19.07.2019 08:30






History, 19.07.2019 08:30





