
Chemistry, 03.11.2021 01:20 jabraeshaw
The diagram shows the course of the reaction for a chemical reaction. With and without catalyst. R stands for reactant and p stands for product. Which statement or statements are true?
1. H4 and H5 together make up the total enthalpy change of the reaction.❌?
2. The reaction is endothermic.❌
3. The presence of catalyst allows a larger amount to be formed a larger amount of the products.❌?
4. H1 <0 ✅
(EXOTERM h1 <0
(Endotherm h1> 0)
5. The activation energy is higher for the catalyzed reaction ❌?
6. The reaction is exothermic
7. H1 is the total enthalpy change ✅?
Activation energy non-catalyzed reaction = H3? ✅
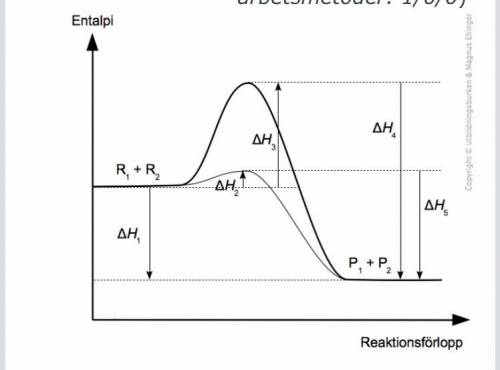

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
What forms when chemical reactions combine pollution with sunlight?
Answers: 1


Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1
You know the right answer?
The diagram shows the course of the reaction for a chemical reaction. With and without catalyst. R s...
Questions

English, 23.06.2021 17:50

Business, 23.06.2021 17:50




English, 23.06.2021 17:50

Computers and Technology, 23.06.2021 17:50


Mathematics, 23.06.2021 17:50






Mathematics, 23.06.2021 17:50







