
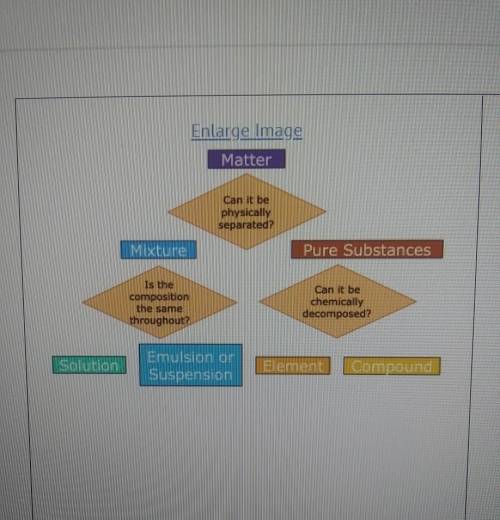
A student has a sample of ocean water that they take to science class. what steps could be taken to determine to classify the ocean water as a suspension, solution, element, or compound?
A) Examine the pH of the sample. If the pH is exactly neutral, it is a pure substance that is a compound.
B) Evaporate the water. If salt is left behind, the sample is a mixture that can be defined as a solution.
C) Let the sample settle. If the salt settles to the bottom, it is a mixture that is classified as a solution.
D) Observe the diffraction of light as it moved through the sample. If light does not scatter, it is a pure substance that is elemental.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:30
The number of moles of a given mass of a substance can be found without knowing its molecular formula or molar mass. true false
Answers: 1


Chemistry, 23.06.2019 06:00
Give one example of a pure (exact) number and of an estimated (measured) number.
Answers: 2

Chemistry, 23.06.2019 11:40
Which of the following would have the lowest average kinetic energy
Answers: 1
You know the right answer?
A student has a sample of ocean water that they take to science class. what steps could be taken to...
Questions

Computers and Technology, 10.03.2020 00:23




History, 10.03.2020 00:23

Social Studies, 10.03.2020 00:23




Computers and Technology, 10.03.2020 00:24


Computers and Technology, 10.03.2020 00:24



Computers and Technology, 10.03.2020 00:24

Computers and Technology, 10.03.2020 00:24






