Emistry
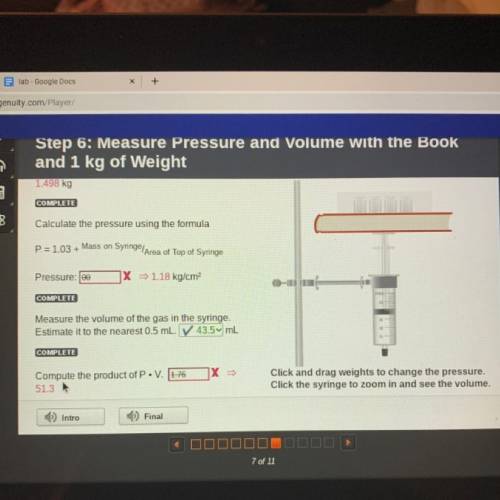
Step 6: Measure Pressure and Volume with the Book
and 1 kg of Weight
1.498 kg<...

Chemistry, 02.12.2021 06:50 EggWithWheels
Emistry
Step 6: Measure Pressure and Volume with the Book
and 1 kg of Weight
1.498 kg
COMPLETE
Calculate the pressure using the formula
P=1.03 + Mass on Syringe Area of Top of Syringe
Pressure: 100
X = 1.18 kg/cm
COMPLETE
Measure the volume of the gas in the syringe.
Estimate it to the nearest 0.5 mL. V 43.5 mL
COMPLETE
Compite the product of P. V. 176
X =
Click and drag weights to change the pressure.
Click the syringe to zoom in and see the volume.
513
Intro
) Final
DO

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3


Chemistry, 22.06.2019 14:30
1) describe the physical layout of the ocean floor ? 2) explain how the dumbo octopus swims differently than other octopus species and why this would be an advantage in the aphonic zone . 3) why are the types of organisms that live at each underwater hot vent so dramatically different ?
Answers: 3

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1
You know the right answer?
Questions




History, 29.09.2019 16:10


Computers and Technology, 29.09.2019 16:10

Biology, 29.09.2019 16:10


Social Studies, 29.09.2019 16:10


Biology, 29.09.2019 16:10

History, 29.09.2019 16:10

Chemistry, 29.09.2019 16:10






Chemistry, 29.09.2019 16:10




