
Chemistry, 09.12.2021 19:40 2337911726
Consider the equations below.
4 equations. 1, Upper C Upper H Subscript 4 Baseline (g) right arrow Upper C (s) + 2 Upper H Subscript 2 Baseline (g) Delta H Subscript 1 Baseline = 74.6 kilojoules. 2, Upper C (s) + 2 Upper C l Subscript 2 Baseline (g) right arrow Upper C Upper Cl Subscript 4 Baseline (g) Delta H Subscript 2 Baseline = negative 95.7 kilojoules. 3, 2 Upper H Subscript 2 Baseline (g) + 2 Upper C l Subscript 2 Baseline (g) right arrow 4 Upper H Upper Cl (g) delta H Subscript 3 Baseline = negative 284.6 kilojoules. 4, Upper C Upper H Subscript 4 Baseline (g) + 4 Upper C l Subscript 2 Baselines (g) right arrow Upper C Upper C l Subscript 4 Baseline (g) + 4 Upper H Upper C L (g) Delta H 4 = negative 205.7 kilojoules.
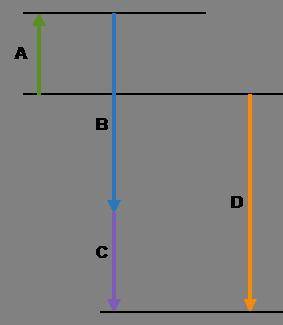
Complete the following based on the diagram.
Arrow A:
Arrow B:
Arrow C:
Arrow D:

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 02:00
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1


You know the right answer?
Consider the equations below.
4 equations. 1, Upper C Upper H Subscript 4 Baseline (g) right arrow...
Questions


Social Studies, 15.07.2019 11:20

Mathematics, 15.07.2019 11:20




Social Studies, 15.07.2019 11:20

Biology, 15.07.2019 11:20

Biology, 15.07.2019 11:20

History, 15.07.2019 11:20



Biology, 15.07.2019 11:20

Business, 15.07.2019 11:20


History, 15.07.2019 11:20

Social Studies, 15.07.2019 11:20



Social Studies, 15.07.2019 11:20



