
Chemistry, 05.01.2022 19:30 natalie857123
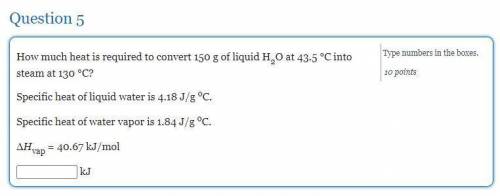
How much heat is required to convert 150 g of liquid H2O at 43.5 °C into steam at 130 °C?
Specific heat of liquid water is 4.18 J/g oC.
Specific heat of water vapor is 1.84 J/g oC.
ΔHvap = 40.67 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Alculate the concentration of h3o⁺in a solution that contains 5.5 × 10-5m oh⁻at 25°c. identify the solution as acidic, basic, or neutral.a) 1.8 × 10-10m, basicb) 1.8 × 10-10m, acidicc) 5.5 × 10-10m, neutrald) 9.2 × 10-1m, acidice) 9.2 × 10-1m, basic
Answers: 1

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 06:00
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3
You know the right answer?
How much heat is required to convert 150 g of liquid H2O at 43.5 °C into steam at 130 °C?
Specific...
Questions



History, 29.01.2021 18:00

Mathematics, 29.01.2021 18:00

Social Studies, 29.01.2021 18:00




World Languages, 29.01.2021 18:00




Mathematics, 29.01.2021 18:00


History, 29.01.2021 18:00


Mathematics, 29.01.2021 18:00





