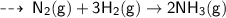Chemistry, 30.01.2022 01:00 SKYBLUE1015
The balanced chemical equation for the reaction of nitrogen with hydrogen to produce
Ammonia gas is-
A. N2(g) + H2(g) → 2NH3(g) B. 2N2(g) + H2(g) → 2NH3(g)
C. N2(g) + 3H2(g) → 2NH3(g) D. 2N2(g) + H2(g) →2NH3(g)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:40
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration
Answers: 1

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 14:30
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1
You know the right answer?
The balanced chemical equation for the reaction of nitrogen with hydrogen to produce
Ammonia gas i...
Questions

Geography, 30.06.2021 14:00



English, 30.06.2021 14:00

SAT, 30.06.2021 14:00

Computers and Technology, 30.06.2021 14:00


Mathematics, 30.06.2021 14:00




Business, 30.06.2021 14:00

Business, 30.06.2021 14:00

Social Studies, 30.06.2021 14:00






English, 30.06.2021 14:00