
Chemistry, 24.02.2022 01:00 samantha636
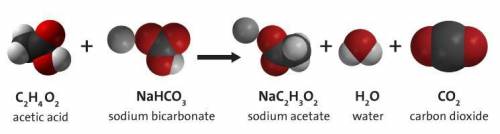
Carbon dioxide gas can be prepared by the reaction of acetic acid and sodium
If 94.46g of sodium bicarbonate are completely reacted, how many liters of carbon dioxide are produced?
Remember to use 1 decimal place when calculating molar mass.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1
You know the right answer?
Carbon dioxide gas can be prepared by the reaction of acetic acid and sodium
If 94.46g of sodium b...
Questions

Mathematics, 18.01.2021 04:10





Advanced Placement (AP), 18.01.2021 04:10



Mathematics, 18.01.2021 04:10



Mathematics, 18.01.2021 04:10



Mathematics, 18.01.2021 04:10

Mathematics, 18.01.2021 04:20

SAT, 18.01.2021 04:20


Mathematics, 18.01.2021 04:20



