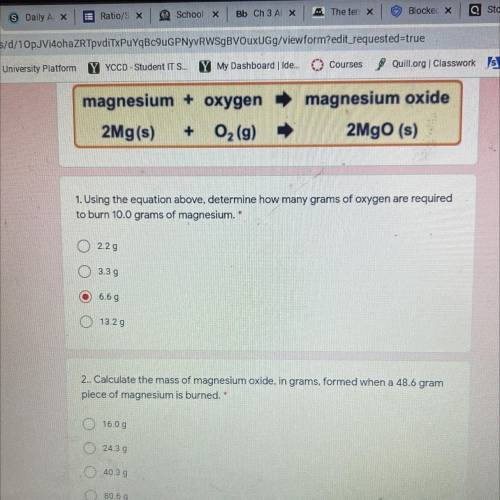
Magnesium + oxygen
2Mg(s) + O2(9)
magnesium oxide
2Mgo (s)
1. Using the equation...

Magnesium + oxygen
2Mg(s) + O2(9)
magnesium oxide
2Mgo (s)
1. Using the equation above, determine how many grams of oxygen are required
to burn 10.0 grams of magnesium. *
2.29
O 3.39
O
6.6 g
O 13.29
2.. Calculate the mass of magnesium oxide, in grams, formed when a 48.6 gram
piece of magnesium is burned. *
O 16.00
O24.39
O 40.39
80.69

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Endeleev saw trends in the physical and chemical properties of elements when he organized them by
Answers: 2

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 22.06.2019 17:30
Energy defines the different "states" of matter. in no more than 3 sentences, describe the amount of kinetic energy that each of the 3 states of matter possesses and relate that to the atom/molecular motion of each "state".
Answers: 2

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
Questions

Business, 23.10.2019 19:00

Mathematics, 23.10.2019 19:00




Mathematics, 23.10.2019 19:00

History, 23.10.2019 19:00



Physics, 23.10.2019 19:00

Mathematics, 23.10.2019 19:00





Mathematics, 23.10.2019 19:00






