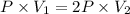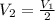Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:30
Clyde and marilyn are riding a roller coaster. during which section(s) of the track is their potential energy converted to kinetic energy? a. from point b to point c only b. from point b to point d only c. from point a to point b only d. from point a to point b and from point c to point d
Answers: 1

Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 22:40
Covalent bonds generally form when the bonded elements have a difference in electronegativity less than 1.5. subtract the electronegativities for the following pairs of elements and predict whether they form a covalent bond. electronegativity difference of c and c: ionic covalent electronegativity difference of mg and cl: ionic covalent
Answers: 1

Chemistry, 23.06.2019 00:00
Exit what is the density of an object having a mass of 5.0 g and a volume of 45.0 cm3?
Answers: 1
You know the right answer?
Consider an ideal gas with a volume of v1. to what volume would you need to compress the gas to doub...
Questions

Mathematics, 05.05.2020 18:12

Business, 05.05.2020 18:12

Biology, 05.05.2020 18:12



History, 05.05.2020 18:12



Mathematics, 05.05.2020 18:12

Mathematics, 05.05.2020 18:12

History, 05.05.2020 18:12

Mathematics, 05.05.2020 18:12



Mathematics, 05.05.2020 18:12

Biology, 05.05.2020 18:12




Social Studies, 05.05.2020 18:12

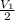
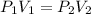
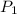 = initial pressure of gas= P atm
= initial pressure of gas= P atm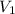 = initial volume of gas =
= initial volume of gas = 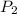 = final pressure of gas= 2P
= final pressure of gas= 2P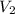 = final volume of gas = ?
= final volume of gas = ?