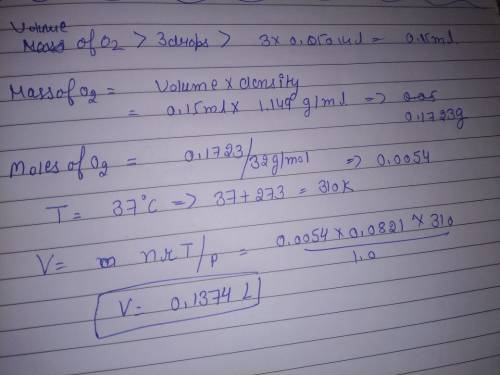Chemistry, 19.07.2019 16:50 growingideas
Aperson accidentally swallows three drops of liquid oxygen, , which has a density of 1.149 g/ml. assuming the drop has a volume of 0.050 ml, what volume of gas will be produced in the person's stomach at body temperature (37 â°c) and a pressure of 1.0 atm?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:00
An aqueous solution of hydroiodic acid is standardized by titration with a 0.186 m solution of calcium hydroxide. if 26.5 ml of base are required to neutralize 20.3 ml of the acid, what is the molarity of the hydroiodic acid solution? m hydroiodic acid
Answers: 1

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 11:00
The diagram below shows the different phase transitions that occur in matter. which arrow represents the transition in which dew is formed?
Answers: 1

You know the right answer?
Aperson accidentally swallows three drops of liquid oxygen, , which has a density of 1.149 g/ml. ass...
Questions





Mathematics, 03.03.2020 06:02








Computers and Technology, 03.03.2020 06:03


English, 03.03.2020 06:03