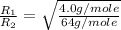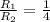Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1


Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
You know the right answer?
How does the rate of effusion of sulfur dioxide, so2, compare to that of helium (he)? (note: the m...
Questions

Mathematics, 25.06.2019 14:00









Mathematics, 25.06.2019 14:00



Business, 25.06.2019 14:00

Health, 25.06.2019 14:00

History, 25.06.2019 14:00


Mathematics, 25.06.2019 14:00



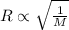
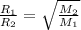
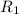 = rate of effusion of sulfur dioxide gas
= rate of effusion of sulfur dioxide gas
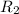 = rate of effusion of helium gas
= rate of effusion of helium gas
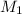 = molar mass of sulfur dioxide gas
= molar mass of sulfur dioxide gas
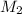 = molar mass of helium gas
= molar mass of helium gas