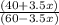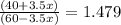Chemistry, 28.07.2019 07:00 kitkat033157
Suppose you have 1.00 l of an aqueous buffer containing 60.0 mmol acetic acid (pka = 4.76) and 40.0 mmol acetate. what volume of 3.50 m naoh would be required to increase the ph to 4.93?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Select the correct answer. which statement is true about a polarized object? o a. it gains electrons and becomes negatively charged. ob. it gains protons and becomes positively charged. oc. the number of positive and negative charges can be the same. od. it has to be a metal. o e. there is no change in the distribution of the charge in the object. reset next what
Answers: 3


Chemistry, 22.06.2019 22:30
The vapor pressure of ethanol is 1.00 × 102 mmhg at 34.90°c. what is its vapor pressure at 61.61°c? (δhvap for ethanol is 39.3 kj/mol.)
Answers: 2

Chemistry, 23.06.2019 06:30
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1
You know the right answer?
Suppose you have 1.00 l of an aqueous buffer containing 60.0 mmol acetic acid (pka = 4.76) and 40.0...
Questions




Health, 01.07.2019 02:40

Mathematics, 01.07.2019 02:40


Mathematics, 01.07.2019 02:40



History, 01.07.2019 02:40

Biology, 01.07.2019 02:40

Mathematics, 01.07.2019 02:40

English, 01.07.2019 02:40



Mathematics, 01.07.2019 02:40


History, 01.07.2019 02:40


![\frac{[CH_{3}COO^-]}{[CH_3COOH]}](/tpl/images/0141/9497/e74fb.png)