Chemistry, 23.07.2019 21:00 janelisse199820
The combustion of gasoline produces carbon dioxide and water. assume gasoline to be pure octane (c8h18) and calculate the mass (in kg) of carbon dioxide that is added to the atmosphere per 1.3 kg of octane burned. (hint: begin by writing a balanced equation for the combustion reaction.)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2


Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 16:30
4. a 20-kg child is tossed up into the air by her parent. the child is 2 meters off the ground traveling 5 m/s. circle one: ke / gpe / both show your work for finding the values of each type of energy the object has:
Answers: 1
You know the right answer?
The combustion of gasoline produces carbon dioxide and water. assume gasoline to be pure octane (c8h...
Questions



Biology, 07.05.2021 15:40

Mathematics, 07.05.2021 15:40



Biology, 07.05.2021 15:40

Mathematics, 07.05.2021 15:40

Biology, 07.05.2021 15:40




Mathematics, 07.05.2021 15:40





Chemistry, 07.05.2021 15:40

English, 07.05.2021 15:40

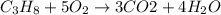
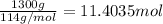
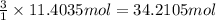 of carbon dioxide
of carbon dioxide