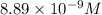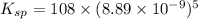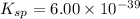Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1
You know the right answer?
The molar solubility of ba3(po4)2 is 8.89 x 10-9 m in pure water. calculate the ksp for ba3(po4)2. t...
Questions


Mathematics, 16.01.2020 20:31



Business, 16.01.2020 20:31





Mathematics, 16.01.2020 20:31






Mathematics, 16.01.2020 20:31



Biology, 16.01.2020 20:31

Physics, 16.01.2020 20:31

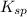 is
is 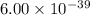
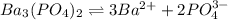
![K_{sp}=[Ba^{2+}]^3[PO_4^{3-}]^2](/tpl/images/0124/1385/f39cc.png)
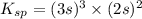
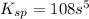
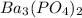 = s =
= s =