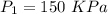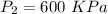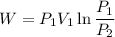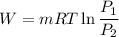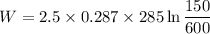Engineering, 05.10.2019 13:30 jpsaad00
2.5 kg of air at 150 kpa and 12°c is contained in a gas-tight, frictionless piston-cylinder device. the air is now compressed to a final pressure of 600 kpa. during the process, heat is transferred from the air such that the temperature inside the cylinder remains constant. calculate the work input during this process.

Answers: 1


Another question on Engineering

Engineering, 04.07.2019 18:10
Assuming compressible flow of air and that the measurements are done at flagstaff a pitot static tube that gives the difference of total and static pressure measures 0.35 m of mercury. what is the velocity of air? assume the temperature to be 300k. (submit your excel or matlab calculation sheet)
Answers: 1

Engineering, 04.07.2019 19:20
The power source in a certain welding setup generates 3500w that is transferred to the low carbon steel work with a heat transfer factor of 0.85. the melting factor in the operation is 0.45. a continuous fillet weld is to be made with a cross-sectional area of 23 mm2 determine the travel speed at which the welding can be accomplished.
Answers: 3

Engineering, 04.07.2019 19:20
A5 kg block of fe is dropped into a very large vat of water. the fe and water initial temperatures are 95 and 25 c, respectively. the fe final temperature is 25 c and the water can be treated as a thermal reservoir,. treated as a thermal reservoir take the water to be the system and determine the entropy generation. report vour answer in kj/k.
Answers: 1

Engineering, 05.07.2019 04:30
Technician a says that in a worm gear steering system, most excessive steering free play is usually found in the gearbox. technician b says that in a rack-and-pinion steering system, excessive free play can be found in the bushings. who is correct?
Answers: 2
You know the right answer?
2.5 kg of air at 150 kpa and 12°c is contained in a gas-tight, frictionless piston-cylinder device....
Questions

Social Studies, 09.07.2019 17:00

Social Studies, 09.07.2019 17:00








Business, 09.07.2019 17:00

History, 09.07.2019 17:00

Mathematics, 09.07.2019 17:00

Biology, 09.07.2019 17:00



History, 09.07.2019 17:00


Mathematics, 09.07.2019 17:00