Mathematics, 08.08.2019 20:20 joeyhd
The enthalpy of vaporization of water ah = kj mol^-1, at t = when water ㎝3 in an open vessel evaporates at this temperature, (a) calculate the change in entropy of the surroundings; 1 mark] (b) has the entropy of the surroundings increased or decreased? [1 mark]

Answers: 1


Another question on Mathematics


Mathematics, 21.06.2019 18:00
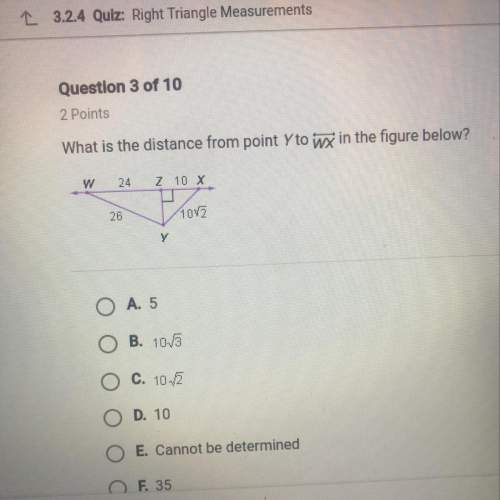
Need on this geometry question. explain how you did it.
Answers: 2

Mathematics, 21.06.2019 18:00
1. a parachutist is 800 feet above the ground when she opens her parachute. she then falls at a constant rate of 5 feet per second. select the equation that represents this situation. h = -800t + 5 y = -5x + 800 h = 5t - 800 y = 800x + 5 i need
Answers: 1

You know the right answer?
The enthalpy of vaporization of water ah = kj mol^-1, at t = when water ㎝3 in an open vessel evapor...
Questions



History, 31.07.2019 19:30



Biology, 31.07.2019 19:30


Health, 31.07.2019 19:30




Mathematics, 31.07.2019 19:30






Mathematics, 31.07.2019 19:30


Mathematics, 31.07.2019 19:30