

Answers: 1


Another question on Physics

Physics, 22.06.2019 08:30
A17,250 kg rocket is pushed with a thrust of 6,450,284 n. what is the acceleration of the rocket?
Answers: 1

Physics, 22.06.2019 11:00
What is the rate of 12 liters of water moving through a water hose in 4.0 minutes?
Answers: 1

Physics, 22.06.2019 12:50
Match each vocabulary term to its definition. 1. electrons neutral subatomic particles found in the nucleus of the atom 2. neutron lowest energy position of an electron in an atom 3. photon the path of an electron around the nucleus of an atom 4. ground state negatively charged, subatomic particles 5. protons packet of energy of specific size 6. element substance with only one type of atom 7. orbital positively charged, subatomic particles found in the nucleus of the atom
Answers: 1

You know the right answer?
Determine the wavelength of incident electromagnetic radiation required to cause an electron transit...
Questions

Computers and Technology, 01.11.2020 14:00

Mathematics, 01.11.2020 14:00

Social Studies, 01.11.2020 14:00

English, 01.11.2020 14:00


Mathematics, 01.11.2020 14:00


Health, 01.11.2020 14:00

Social Studies, 01.11.2020 14:00

Computers and Technology, 01.11.2020 14:00

Mathematics, 01.11.2020 14:00




Spanish, 01.11.2020 14:00

Mathematics, 01.11.2020 14:00



Mathematics, 01.11.2020 14:00

History, 01.11.2020 14:00

![\frac{1}{\lambda} = R[ \frac{1}{n_1^2} - \frac{1}{n_2^2} ]](/tpl/images/0110/4724/93668.png) --- (1)
--- (1)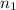 = 5
= 5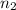 = 7
= 7![\frac{1}{\lambda} = (1.096 * 10^7)[ \frac{1}{5^2} - \frac{1}{7^2} ]](/tpl/images/0110/4724/8f96d.png)
![\frac{1}{\lambda} = (1.096 * 10^7)[ 0.04 - 0.020 ] \\ \lambda = \frac{1}{(1.096 * 10^7)[0.020 ]} \\ \lambda = 4562 nm](/tpl/images/0110/4724/aebbc.png)


