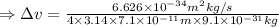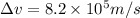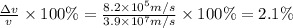Physics, 29.06.2019 04:00 avalonr2003
An atom of argon has a radius of 71.pm and the average orbital speed of the electrons in it is about ×3.9107/ms. calculate the least possible uncertainty in a measurement of the speed of an electron in an atom of argon. write your answer as a percentage of the average speed, and round it to 2 significant digits.

Answers: 1


Another question on Physics

Physics, 22.06.2019 04:30
Which of the following are not typically included in the periodic table? a. atomic mass b. element symbol c. isotopes d. number of electrons
Answers: 2

Physics, 22.06.2019 11:10
Avolcano erupts next to a grassland area in a valley. a. describe three ways in which material released by the volcano could impact the grassland area. b. describe three ways in which the grassland ecosystem could recover after a volcanic eruption.
Answers: 3

Physics, 22.06.2019 12:30
Which governments provide garbage collection services to homes and businesses
Answers: 3

Physics, 22.06.2019 14:00
Select for each of the following statements whether it is correct or incorrect. (a) in an isothermal expansion of an ideal gas. (b) the temperature remains constant. (b) the pressure remains constant. (c) there is work done by the gas. (d) there is heat added to the gas. (e) the change in internal energy equals zero.
Answers: 1
You know the right answer?
An atom of argon has a radius of 71.pm and the average orbital speed of the electrons in it is about...
Questions



Physics, 22.06.2019 00:30



Mathematics, 22.06.2019 00:30



History, 22.06.2019 00:30

Biology, 22.06.2019 00:30



English, 22.06.2019 00:30

English, 22.06.2019 00:30



Mathematics, 22.06.2019 00:30