
Physics, 24.11.2019 21:31 anaalashay
A3-kg object experiences a temperature rise of 50°c when 1000 joules of heat are added. using the same substance, which situation would require the same amount of heat to be added? a) a 6-kg mass experiences a temperature rise of 50°c. b) a 3-kg mass experiences a temperature rise of 50°c. c) a 6-kg mass experiences a temperature rise of 100°c. d) a 3-kg mass experiences a temperature rise of 100°c.

Answers: 2


Another question on Physics

Physics, 22.06.2019 05:30
Acombination reaction is when two or more combine to form one product. a decomposition reaction is when a substance breaks down into two or more simpler substances in a chemical reaction.
Answers: 1

Physics, 22.06.2019 12:30
Consider a 1000 w iron whose base plate is made of 0.5 cm thick aluminum alloy 2024-t6 (ρ = 2770 kg/m3 and cp = 875 j/kg°c). the base plate has a surface area of 0.03 m2. initially, the iron is in thermal equilibrium with the ambient air at 22°c. assuming 90% of the heat generated in the resistance wires is transferred to the plate, determine the minimum time needed for the plate temperature to reach 200°c.
Answers: 1

Physics, 22.06.2019 14:40
You throw a small rock straight up from the edge of a highway bridge that crosses a river. the rock passes you on its way down, 7.00 s after it was thrown. what is the speed of the rock just before it reaches the water 28.0 m below the point where the rock left your hand? ignore air resistance.
Answers: 2

Physics, 22.06.2019 15:30
Two pans of a balance are 24.1 cm apart. the fulcrum of the balance has been shifted 1.33 cm away from the center by a dishonest shopkeeper. by what percentage is the true weight of the goods being marked up by the shopkeeper? assume the balance has negligible mass. answer in units of %.
Answers: 1
You know the right answer?
A3-kg object experiences a temperature rise of 50°c when 1000 joules of heat are added. using the sa...
Questions


Mathematics, 23.08.2021 20:20



Advanced Placement (AP), 23.08.2021 20:20














Computers and Technology, 23.08.2021 20:20

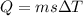

 = temperature rise
= temperature rise

