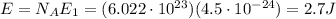For the wave of light you generated in the part b, calculate the amount of energy in 1.0 mol of photons with that same frequency (6.8×109 hz ) and wavelength (0.044 m ). recall that the avogadro constant is 6.022×1023 mol−1. express the energy in joules to two significant figures.

Answers: 2


Another question on Physics

Physics, 21.06.2019 14:00
How much heat is required to convert 0.3 kg of ice at 0°c to water at the same temperature
Answers: 1

Physics, 21.06.2019 22:00
Bernie drove home from a vacation in 1.5 hours. he traveled a total distance of 60 miles. what was his average speed in miles per hour? a. 60 mph b. 90 mph c. 40 mph d. 55mph
Answers: 1

Physics, 22.06.2019 13:40
Dao makes a table to identify the variables used in the equations for centripetal acceleration. what quantities belong in cells x and y?
Answers: 2

Physics, 22.06.2019 15:00
10 points! will mark brainiest! in a heat engine if 1,000 j of heat enters the system and the piston does 500 j of work, what is the final internal energy of the system if the initial energy was 2,000 j 1: write the equation 2: list out your known variables 3: plug the numbers into the equations 4: solve 5: write your solution statement that includes initial energy and final energy added you so much!
Answers: 3
You know the right answer?
For the wave of light you generated in the part b, calculate the amount of energy in 1.0 mol of phot...
Questions



Mathematics, 31.01.2020 11:56




Mathematics, 31.01.2020 11:56







Physics, 31.01.2020 11:56




Social Studies, 31.01.2020 11:56


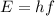
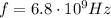
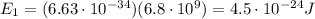
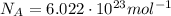 (Avogadro number)
(Avogadro number)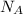 photons contained in 1.0 mol is
photons contained in 1.0 mol is