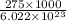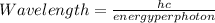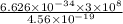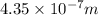Physics, 16.09.2019 20:30 nayiiii1874
The energy required to dislodge electrons from sodium metal via the photoelectric effect is 275 kj/mol . what wavelength of light, in nanometers, has sufficient energy per photon to dislodge an electron from the surface of sodium? express the wavelength in nanometers to three significant figures.

Answers: 1


Another question on Physics

Physics, 21.06.2019 22:00
What type of light does this light bulb produce most (i.e. at what wavelength does the spectrum have maximum intensity)?
Answers: 3

Physics, 22.06.2019 03:00
Ahot-air balloonist, rising vertically with a constant speed of 5.00 m/s releases a sandbag at the instant the balloon is 40.0 m above the ground. after it is released, the sandbag encounters no appreciable air drag. compute the velocity of the sandbag at 0.250 s after its release.
Answers: 2

Physics, 22.06.2019 04:30
Ameter stick is pivoted at the 0.50-m line. a 3.0-kg object is hung from the 0.15-m line. where should a 5.0-kg object be hung to achieve equilibrium (the meter stick oriented horizontal and motionless)?
Answers: 1

Physics, 22.06.2019 06:40
Light traveling in a medium with a refractive index 1.19 is incident on a plate of another medium with index of refraction 1.79. at what angle of incidence is the reflected light fully polarized?
Answers: 2
You know the right answer?
The energy required to dislodge electrons from sodium metal via the photoelectric effect is 275 kj/m...
Questions

Physics, 20.12.2020 18:10



Mathematics, 20.12.2020 18:10

Social Studies, 20.12.2020 18:10

Biology, 20.12.2020 18:10

Mathematics, 20.12.2020 18:10


Mathematics, 20.12.2020 18:10

Mathematics, 20.12.2020 18:10

Physics, 20.12.2020 18:10

Business, 20.12.2020 18:10


Mathematics, 20.12.2020 18:20



English, 20.12.2020 18:20



Spanish, 20.12.2020 18:20